Products
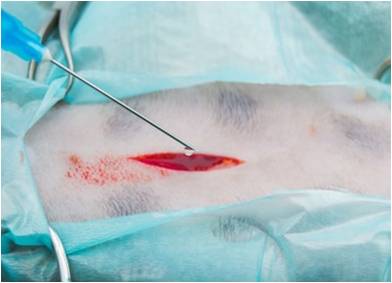
Hemostasis
During a surgical process, massive bleeding beyond control is life-threatening. In addition to affecting the doctor's visual field during surgery and prolonging the operation time, it also increases the unpredictable risk to the patient, and in severe cases, it may even lead to death. Therefore, local rapid hemostasis of trauma is very important throughout any surgery. Our company expects to develop a biodegradable anti-adhesion hemostatic spray to facilitate local hemostasis in clinical surgery.
|
Bio-glue
In recent years, traditional surgical suture methods including sutures and surgical staplers have been unable to meet the needs of physicians in some operations. Biomedical technology is committed to developing a high-tech wound closure systems such as hemostatic agents, tissue adhesives or bio-adhesives.
|
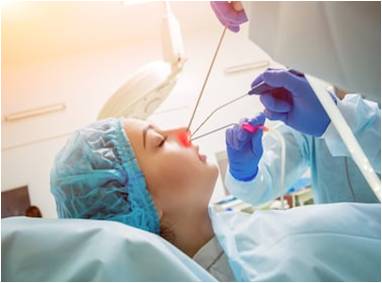
Anti-Adhesion
Anti-epidemic of nasal mucosa sprayIn response to the epidemic situation caused by new coronavirus, we developed a product with both moisturizing and antibacterial properties. After spraying from the nasal cavity, it quickly formed a bio-membrane on the nasal mucosa to achieve the purpose of epidemic blocking and prevention.
|
Postoperative anti-adhesionAfter surgery, the wound in the body often produces exudate or blood during the healing process, which is easy to form adhesions with adjacent tissues, and leads to occurrence of related complications such as intestinal obstruction, abdominal and pelvic pain, etc. The most ideal way to prevent such complications is to use a physical barrier to isolate the wound from adjacent organs.
|